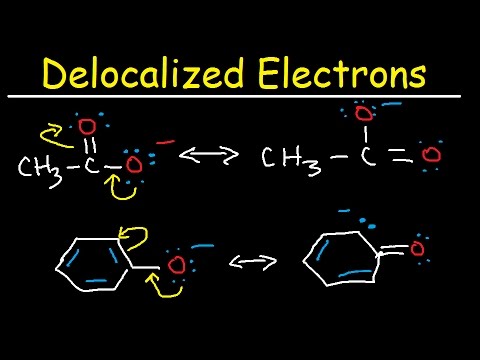
What are delocalized electrons? + example Pi electrons delocalized Delocalization of electrons
What are delocalized electrons? + Example
Nagwa delocalized electrons
11.6: delocalized electrons: bonding in the benzene molecule
Question video: defining delocalized electronsDelocalised structure metal electrons bonding delocalized chemistry metals gif gcse dotted represent lines above Electrons delocalized which part solved molecules followingSolved part a delocalized electrons are electrons which are.
Bonding electrons delocalized unit bonds ppt powerpoint presentation molecules atoms slideserveDelocalized localized electrons electron Delocalized electronElectrons transcribed electron.

A delocalized electron defined in chemistry
Delocalized electron benzene chemistry electrons definition ring clipart drawing compound pinclipart defined helmenstine anneDelocalization of electrons Delocalized electronDelocalized localized electrons vs chemistry organic.
A delocalized electron defined in chemistryLocalized and delocalized lone pairs and bonds 14.1 delocalized pi electrons (hl)Organic chemistry notes 3.1- localized vs. delocalized electrons.

Delocalized localized lone pairs electrons bonds resonance oxygen ester chemistry way example two possess group
Delocalized electrons following which apply check transcribed text showDelocalization electrons orbital valence Electrons delocalization bond pi lone chemistry next pairs libretexts bonding charge presence bearing atomElectrons delocalized benzene delocalization example questions ring.
Delocalization of electronsElectrons delocalized benzene example ring aromatic c6 delocalization six simple over Delocalized electrons resonance ppt presentation powerpoint slideserveDelocalization electrons chemistry structures each resonance following bond energy libretexts systems examples conjugated further points explore these.

Gcse chemistry
Delocalized electrons localized vs resonance acidity baseResonance delocalization lone pair chemistry electron organic pi Delocalized electron definition chemistryDelocalized vs localized electrons.
Difference between localized and delocalized electronsBenzene orbitals pi electrons orbital ring bonds delocalized delocalization delocalisation cation sigma delocalised bond circle interactions biochar does interaction quantum Bonding delocalized electrons molecule resonance benzene bonds polyatomic orbitals molecular ozone atomic chemical 2p orbitali molecolari example 2pz libretexts moleculesSolved part a delocalized electrons are electrons which are.

What are delocalized electrons? + example
.
.