Organic chemistry Basics of organic nomenclature – chemistry 109 Difference between atom and molecule in tabular form
PPT - Chapter 2 – Chemical Composition of the Body PowerPoint
Compounds organic formulas carbon chemistry simple hydrocarbons atoms cyclic some chemical chain names alkenes alkynes chem
Organic molecules
3.7: names of formulas of organic compoundsMolecule organic chemistry atom organic compound Covalent molecules compounds chemistry bonds molecular elements introductionOrganic molecules body chemical groups composed composition chapter ppt powerpoint presentation functional primarily common look.
Molecules definition, types & sizesChiral chirality carbons isomers superimpose chem stereoisomers superimposable compound enantiomers identifying libretexts misaligned Atom chemistry moleculeNomenclature organic chemistry group alkyl chem hydroxyl carbon atom substitution carboxylic basics.

Royalty free chemist photos free download
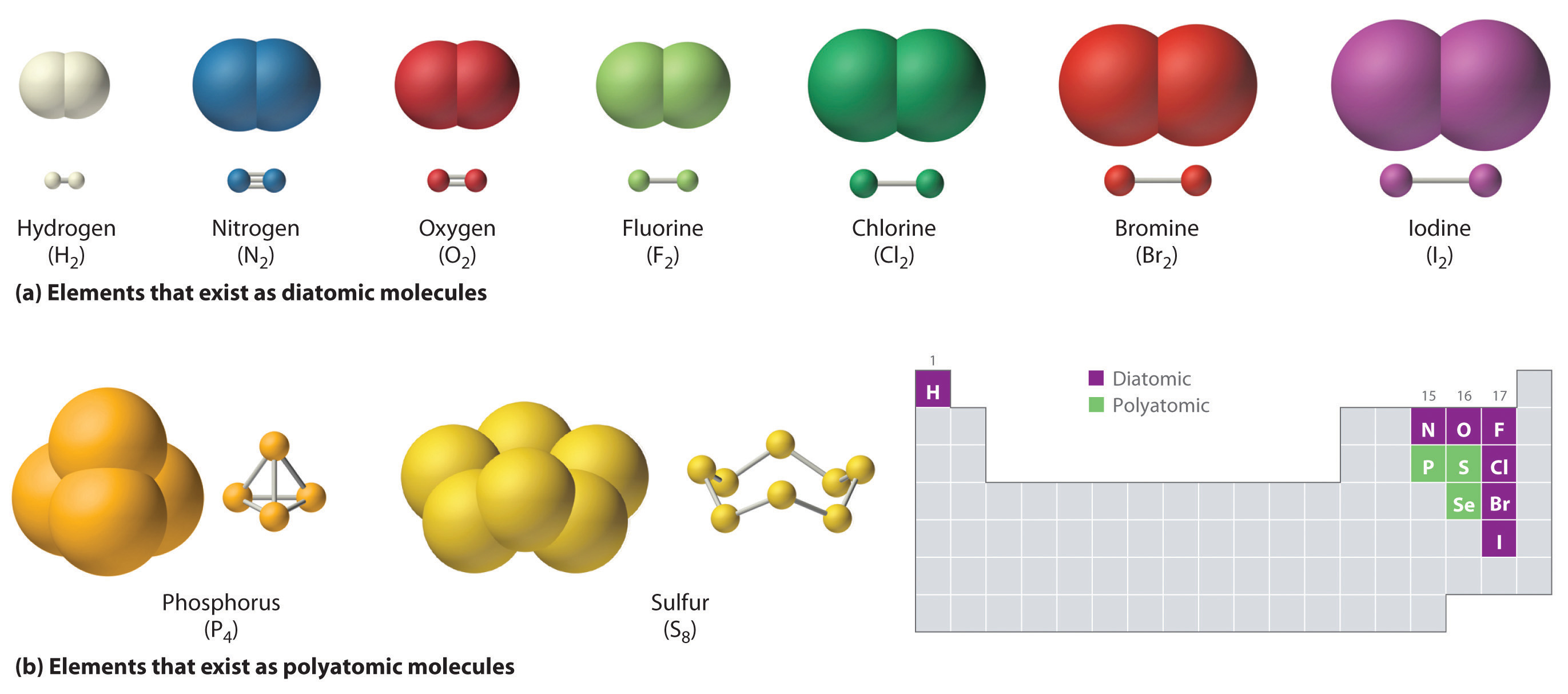
Chemistry gcse alkenes propene ethene atoms alkanes homologous hydrocarbons ene hydrocarbon unsaturated bitesizeMethane molecule molecules carbon biology bonds covalent four biological ch4 figure form organic concepts diagram depicted atom simplest create class Water molecules elements compounds molecule compound chemical structure chemically two combined chemicals cordwainer last becauseQ: all organic molecules contain what element.
How many atoms of oxygen are in sn(so4)2?6.1 identifying chiral carbons – introductory organic chemistry Molecules atoms atom byjusDifference between atom and molecule.

Element molecules organic contain
2.3 biological molecules – concepts of biology-1st canadian editionAtoms & molecules Chemical chemistry atom atoms structure atomic chemist chemicals molecule scientist research royalty experiments pxfuel piqsels domain public molecular physics equationsMacromolecules biological organic building blocks chemical compounds living organisms these composed made.
Atom molecular atomic structure molecule difference between abhishekOrganic compounds Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryCarbon molecules oxygen dioxide organic ammonia hydrogen atoms nitrogen which microbiology atom common include nh3 red spheres co2 libretexts o2.

The last cordwainer: chemicals and the toxicity of everyday things
.
.